The physiology of sleep will help you understand the root causes of sleep problems and how you should approach them. In this post, we cover aspects of sleep physiology, focusing on the roles of neurotransmitters and other brain-derived factors in sleep control.
Sleep and Neurotransmitters
The neural control of sleep is like a seesaw between the sleep and wakefulness state, which is controlled by orexin neurons.
During sleep, neurons in the hypothalamus produce sleep neurotransmitters such as GABA and galanin and inhibit dopamine, histamine, norepinephrine and serotonin neurons (monoaminergic neurons).
During wakefulness, neurons secrete dopamine, histamine, norepinephrine, and epinephrine while inhibiting GABA.
1) Orexin/Hypocretin
Orexin, also called hypocretin, is a neuropeptide that increases arousal, wakefulness, and appetite. Orexin-producing neurons work during active wakefulness and stop during sleep [1].
The importance of orexin in the maintenance of sleep/wake cycle was first demonstrated by the fact that orexin deficiency caused narcolepsy in humans and animals [2, 3, 4, 5].
A lack of orexins correlates with narcolepsy. Approximately 90% of patients with narcolepsy (with cataplexy) show decreased orexin-A levels in the cerebrospinal fluid (CSF, the fluid surrounding the brain) [6].
Mice lacking the gene for orexin showed phenotypes similar to humans with narcolepsy and disruption of the hypocretin receptor 2 (Hcrtr2) caused canine narcolepsy [2, 7, 4].
Both noradrenaline and serotonin can inhibit the activity of orexin neurons [8, 9].
Loss of function in the orexin neurons is also involved in altered energy homeostasis, which could result in fatigue [10].
Read this post to learn more about the orexin system and ways to ensure it functions well.
2) Dopamine
Dopamine functions as a neurotransmitter and is crucial for arousal, memory and motor function [11].
Altered brain dopamine leads to several neurological disorders like Parkinson’s, schizophrenia and ADHD [12, 13].
Individuals with these diseases show dramatic sleep disturbances, including excessive daytime sleepiness, rapid-eye sleep movement disorder, decreased REM sleep and disturbed sleep architecture [14, 15].
These observations show that dopamine function and dopamine receptors may play a role in regulating sleep-wake cycles. Generally, high dopamine induces wakefulness while blocking dopamine receptors promotes sleep.
Activation of the D1 dopamine receptor increases wakefulness and reduces slow-wave sleep and REM sleep [16].
Activation of the D2 dopamine receptor can have different effects at various doses. Low doses reduce wakefulness and increase Slow Wave and REM sleep, whereas large doses induce the opposite effect (predominant facilitation of the D2 postsynaptic receptors on the neurons that receive dopamine) [16].
Compounds that block both D1 and D2 receptors reduce wakefulness and increase deep sleep [16].
Activation of the D3 receptor induces sleepiness and sleep in laboratory animals and man [16].
During wakefulness, there’s an increase in dopamine neuron activity, and an enhanced release of dopamine in multiple regions of the brain (VTA, the nucleus accumbens, forebrain) [16].
3) Serotonin
Serotonin is an important neurotransmitter for good mood, controlling appetite, and sleep. There are many serotonin (5-HT1-7) receptors, each of which can affect the brain differently. Serotonin is also a precursor to melatonin, the sleep hormone.
Early studies indicate that serotonin was associated with the initiation and maintenance of sleep, while later studies indicate that serotonin neurons also play a role in inhibiting sleep [17].
Serotonin neurons fire at a steady rate during wakefulness and decrease their firing during slow-wave sleep and virtually stop during REM sleep [18].
Supplementation of 5-HTP (a precursor of melatonin and serotonin, at a dose of 2 mg/kg) every night before bed could reduce the arousal level in children (aged 3 – 10) and induce a long-term improvement against nightmares with an effective reduction rate of 93.5% [19].
In a study with an individual with a genetic mutation that caused serotonin deficiency, he lacked a circadian rhythm and overate. For this person, supplementation with 5-HTP restored his normal circadian rhythm and food intake [20].
A small clinical trial demonstrated that the use of 5-HTP in combination with GABA significantly improves sleep quality in 9 subjects with sleep disorders, in comparison to placebo [21].
Serotonin Receptors that Affect Sleep
5-HT2A is the bad serotonin receptor because its activation can cause insomnia and reduce deep sleep [22].
5-HT7 receptors play an important role in the circadian rhythm, in addition to mood, pain sensation, and control of body temperature [23, 24, 25].
5-HT1A, 5-HT1B, 5-HT2A, and 5-HT7 receptors and serotonin transporters control REM sleep. The administration of activators for these receptors increases wakefulness and decreases sleep (SWS and REM) [26, 27, 22, 28].
4) Norepinephrine
Norepinephrine (noradrenaline) is produced mostly in the locus coeruleus (LC) located in the pons region of the brain.
It is released from the sympathetic nervous system in response to stress. Because the release of norepinephrine affects other organs of the body, it is also referred to as a “stress hormone” [29].
This is why stress and anxiety can cause insomnia or reduce sleep quality.
Norepinephrine neurons in the brain (hypothalamus, medial preoptic area) increase wakefulness and arousal [30, 31, 32, 33, 34].
LC neurons are highly active during wakefulness, slow fire during NREM sleep, and are almost completely inactive during REM sleep [35].
Norepinephrine also plays an active role in cataplexy, a condition characterized by transient weakness or paralysis. Blocking the norepinephrine receptor (α1AR) worsens cataplexy, whereas activation of these receptors decreases the number of attacks [36].
5) Histamine
As a neurotransmitter, histamine promotes wakefulness. In the brain, histamine-releasing neurons are located in the hypothalamus (tuberomammillary nucleus) [37].
Histamine neuron firing ebbs and flows with the circadian rhythm. They are active during the day, stop working during drowsy states before sleep and resume activity at highly vigilant states after waking up [38].
Low brain histamine can contribute to excessive daytime sleepiness or prolonged nighttime sleep (hypersomnolence) [38].
Evidence for the role of histamine in sleep/wakefulness:
- Human narcoleptics have decreased brain histamine [39].
- Inhibitors of the enzyme histamine-N-methyltransferase, which breaks down histamine, increase wakefulness [40].
- Application of histamine into the hypothalamus increases wakefulness and reduces NREM sleep, in a dose-dependent manner. The highest dose produced maximal wakefulness [38].
- Antihistamines increase NREM sleep and reduce REM sleep in humans, cats, and dogs [41, 42, 43].
- Inhibitors of histidine decarboxylase (HDC), which is an enzyme that helps produce histamine, increase fatigue [44].
Although histamine promotes wakefulness through the activation of H1R, H2R, and H4R receptors, H3R has an opposite effect. H3R decreases histamine levels and promotes sleep [38, 45].
6) GABA
GABA is the main inhibitory neurotransmitter in the brain. The activation of GABA(A) receptors favors sleep. More than 20% of all brain neurons produce GABA [46, 47].
There are mainly two classes of receptors: GABA(A) and GABA(B).
Many drugs that treat sleep loss conditions target these GABA receptors, thus promoting sleep. Drugs blocking the GABA(A) receptors have been researched for disorders such as insomnia, epilepsy, and narcolepsy [48, 49].
The GABA(B) receptor blockers increase active brain states such as when we’re awake or in REM sleep (dreaming stage) [46].
After long periods of supplementation with GABA/5HTP, the natural production of GABA receptors increased, which assisted with natural sleep cycles and the promotion of healthy sleep [50].
Brain Factors That Promote Sleep
1) Circadian Rhythm
Human sleep occurs with a circadian periodicity, i.e. we sleep at night and wake during the day. Sleeping at the “wrong circadian time” such as traveling across time zones or shift work confuses our circadian rhythm [51].
When the sleep phase is shifted by sleeping too early or too late, REM sleep readjusts only after several days, while slow-wave sleep (SWS), which is less influenced by circadian factors readjusts immediately [52].
The central circadian clock/the circadian pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Nerves in the SCN control the pineal gland, which synthesizes and releases melatonin [53].
Predictably, melatonin synthesis increases as light decreases and reaches its maximal level between 2:00 and 4:00 a.m. In the elderly, the pineal gland calcifies and less melatonin is produced, perhaps explaining why older people sleep fewer hours and are more often afflicted with insomnia [54].
2) Adenosine
Adenosine is a part of ATP (the energy molecule). The buildup of adenosine throughout the day creates sleepiness, and sufficient levels of adenosine are necessary for good sleep.
Caffeine provokes wakefulness and prevents sleep by blocking adenosine receptors [55].
How Adenosine Makes Us Sleepy
ATP release from astrocytes contributes to the levels of adenosine between cells in the brain. The adenosine reduces synaptic transmission (communication between neurons), thus reduces brain function [56].
Mutant mice that cannot move adenosine into space between brain cells showed a reduced slow-wave sleep and a decrease in the recovery sleep following sleep deprivation [57].
There are 4 known adenosine receptors (A1R, A2aR, A2bR, and A3R), of which A1R and A2aR play a major role in sleep homeostasis (since both of them are expressed at high levels throughout the brain) [58, 59].
A2aR has been associated with inflammatory diseases and neurodegenerative disorders [60].
A2AR receptors promote sleep by:
- inhibiting histamine-releasing neurons [61]
- turning on sleep-active neurons in the VLPO part of the hypothalamus [62]
- increasing acetylcholine release in the pontine reticular formation, which results in increased time in SWS and REM sleep [63].
Adenosine receptor activation decreases wakefulness and increases sleep. Furthermore, molecules that activate adenosine receptors tend to increase the deeper stages of SWS [64, 65].
Further evidence for the role of adenosine in promoting sleep:
- A specific adenosine A1 receptor activator (N6-l-methylcyclopentyl-adenosine) has a sleep-promoting effect [66].
- Deoxycoformycin increases adenosine levels by inhibiting adenosine deaminase [67].
- Administration of adenosine (190 nmol) into the brain puts dogs to sleep [67].
3) Uridine
Uridine induces sleep through uridine receptors in the central nervous system. It binds to the P2Y receptors in areas of the brain which regulate natural sleep [68].
Uridine, when administered through a systematic route (such as in the stomach), may pass through the blood-brain barrier to balance sleep [69].
It enhances both REM and NREM in mice at a dose of 1 pmol [70].
P2Y2 (P2Y2R) is activated with equal potency by ATP and uridine triphosphate (UTP) [71]. The SNP rs1791933 (T allele) in the P2Y2 gene is linked to caffeine-related sleep disturbance [72].
4) Oxidized Glutathione
Glutathione is an important cellular antioxidant in its reduced form (GSH). The oxidized form of glutathione (GSSG) is a sleep inducer. In the mammalian brain, glutathione mainly exists in the form of GSH, which is easily converted to GSSG by a peroxidase enzyme [73, 74].
GSSG significantly enhances REM sleep at a dose of 25 nmol and non-REM sleep at a dose of 20 – 50 nmol [75].
In rats, infusing the brain with a substance that induces oxidative stress (such as t-butyl hydroperoxide) at a low dose can trigger sleep in rats [76].
This data suggests that low levels of oxidation in the brain under the control of an antioxidant system may trigger sleep through the increase of GSSG and other substances in the POAH [77].
5) Inflammatory Cytokines (IL-1beta, TNF-alpha)
Administration of exogenous IL-1beta or TNF-alpha increased non-rapid eye movement sleep (NREMS). Inhibition of IL-1 or TNF-alpha makes it harder to fall asleep [78].
IL-1beta
IL-1beta is a cytokine protein that, in humans, is made from the IL1B gene. It is one of 11 family members with respective receptors termed the interleukin-1 family (IL1F) [79, 80].
The pro-inflammatory IL1F members, IL-1beta, IL-1 alpha, and IL-18 promote NREM sleep while the IL-1 receptor antagonist/blocker (IL-1RA) reduces NREM sleep [81].
The sleep-promoting effect of IL-1beta was first demonstrated in rabbits, administration of IL-1beta enhanced NREM sleep, while an injection of an IL-1beta inhibitor reduced sleep [82, 83].
Injecting IL1-beta increases NREMS in cats, monkeys, and humans [84, 85].
In humans, plasma IL1-beta peaks at the onset of slow-wave sleep, it enhances the firing rate of hypothalamic sleep-active neurons and inhibits wake-active neurons [86, 87].
TNF-alpha
TNF-alpha is a cytokine involved in systemic inflammation. Sleep-inducing effects of TNF-alpha were also first described in rabbits. Injection of TNF-alpha in the blood or in the brain enhances the duration and intensity of NREMS and decreases REMS [88, 89].
Circulating TNFα levels increase with sleep propensity. In humans, TNFα levels are increased in certain medical conditions associated with altered sleep, such as sleep apnea and insomnia [90, 91, 92].
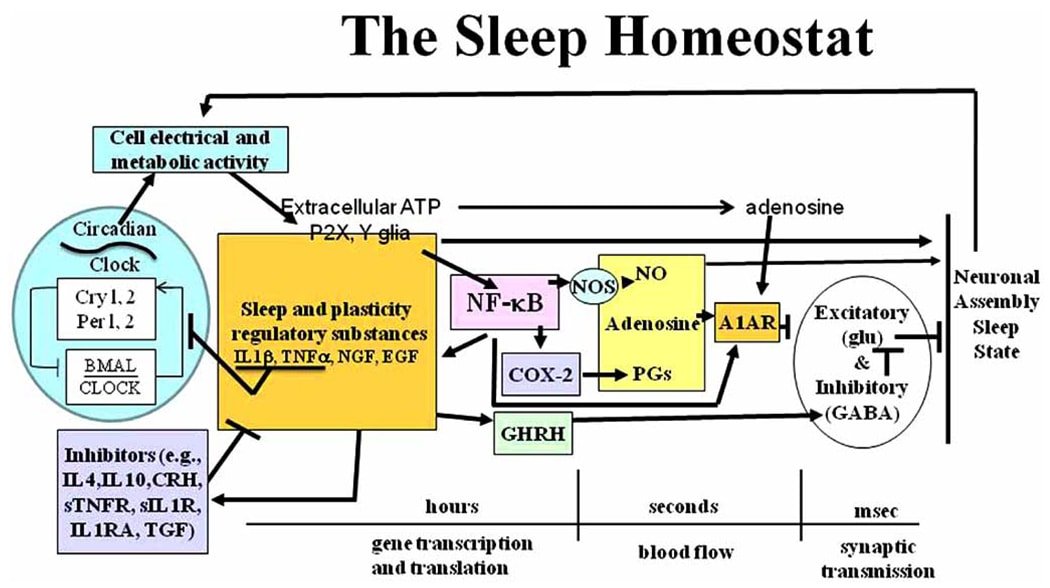
How It Works
Sleep regulatory substances activate a number of inflammatory pathways, including NF-kB, NO (Nitric oxide), and COX (Cyclooxygenase). These inflammatory pathways induce sleep [81].
NF-kB exhibits a diurnal rhythm in the cortex (region of the brain). Sleep deprivation activates NF-kB in the cortex part of the brain. Furthermore, inhibiting NF-kB reduces spontaneous NREM sleep. Both IL-1beta and TNF-alpha promote sleep by activating nuclear factor kappa-B (NF-kB) [93, 94].
Brain iNOS (inducible Nitric Oxide synthase) has a circadian variation and increases with sleep propensity. In rats, iNOS is increased during sleep deprivation. NREM and REM sleep were enhanced after administration of NO precursors, such as L-arginine. TNF-alpha mediates many of its actions via iNOS [95, 96].
Of the two separate COXs (Cyclooxygenases 1 and 2), COX-2 modulates inflammation and sleep. Alternatively, IL-1beta and TNF-alpha induce COX-2 production [81, 97].
Inhibiting COX-2 production reduces spontaneous NREM and TNF-alpha-induced sleep [98, 99].
The figure below shows the various cytokines and their mechanisms as part of a sleep biochemical regulatory network.
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2692603/
6) BDNF
BDNF (Brain-Derived Neurotrophic Factor) is important for the nerve cells to grow. In addition, depressed people have low levels of BDNF, and sleep disturbances are very common among these people [100].
BDNF appears to have a circadian rhythm, being high during the day and lower at night [101].
BDNF production during the day correlates with the amount of slow-wave (deep) sleep during the subsequent night, suggesting that BDNF is a measure of sleep pressure (the body’s desire to sleep) [102].
The T allele of the BDNF SNP called rs6265 correlates with lower BDNF levels and may thus impact sleep quality. In addition, this allele may be associated with cognitive decline [103].
In rats, chronic sleep deprivation led to increased IL-1b and TNF and reduced BDNF [104].
Therefore, BDNF levels may impact sleep quality.



